Multiple Choice
When heat energy is added to a solid and liquid mix at the melting point, the temperature does not increase, as illustrated by horizontal line C. Which of the statements below best describes what happens to the heat energy added to the solid and liquid? 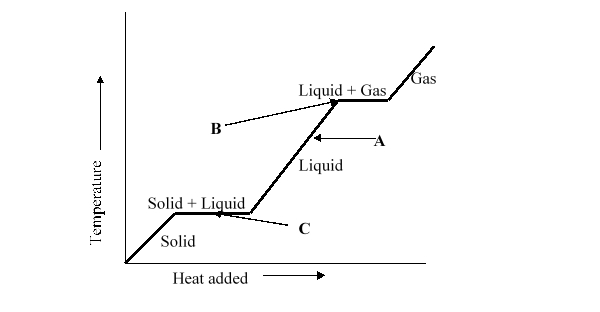
A) It increases the kinetic energy of the molecules.
B) It decreases the kinetic energy of the molecules.
C) It breaks the intermolecular forces between the molecules.
D) It makes new intermolecular forces between molecules.
E) It just passes through the solid and liquid, which is why the temperature does not increase.
Correct Answer:

Verified
Correct Answer:
Verified
Q75: Which statement best describes how heat energy
Q76: What is the electron geometry of the
Q77: The temperature of a gas at 1.00
Q78: Which of the following types of molecules
Q79: Which of the following statements best describes
Q81: What type of change in pressure causes
Q82: Formaldehyde, a common preservative, is shown in
Q83: What is the volume of 2.01 <font
Q85: Which of the following physical change is
Q86: One of the symptoms of the bends