Multiple Choice
Does isopropyl alcohol have a higher or lower boiling point than acetone? 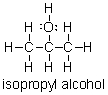
A) higher, because isopropyl alcohol can hydrogen bond
B) lower, because isopropyl alcohol can hydrogen bond
C) higher, because isopropyl alcohol can form dispersion forces
D) lower, because isopropyl alcohol can form dispersion forces
E) It is not possible to determine the answer based on the information provided.
Correct Answer:

Verified
Correct Answer:
Verified
Q10: Which of the following changes of state
Q24: Which has a higher partial pressure of
Q57: Which of the following is the common
Q58: What is the relationship between polarity and
Q59: Which molecule in this table has the
Q61: What is the strongest intermolecular that exists
Q63: Are any bonds in acetone, shown below,
Q64: A sample of gaseous neon has a
Q65: What is the molecular geometry of the
Q67: A liquid has temperature A as shown