Multiple Choice
Imagine that you have a beaker of gas molecules. A small volume of the gas in the beaker is enlarged so you can see the gas particles. If all of the gas in the beaker A is transferred to a new beaker half the size of the original beaker, while maintaining the same temperature, which magnified view best represents what the gas would look like? 
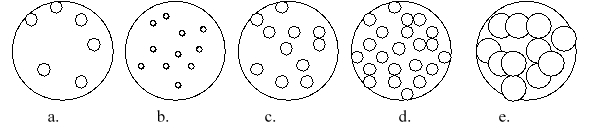
A) a
B) b
C) c
D) d
E) e
Correct Answer:

Verified
Correct Answer:
Verified
Q1: Which bond in acetone, shown below, is
Q3: Which of the following statements best describes
Q4: Three boxes, each containing molecules in the
Q7: It is important to check tire pressure
Q8: Which of the following is the molar
Q9: Which arrow best shows the molecular dipole
Q10: Which statement best describes why it is
Q44: How do phase changes differ from chemical
Q56: Hyperbaric oxygen therapy is the use of
Q98: What happens to the speed of molecules