Multiple Choice
A common, over-the-counter antacid is Al(OH) 3. This antacid reacts with gastric juice (HCl) in the stomach, producing AlCl3 and H2O. Which of the following equations can be used to correctly determine how much gastric juice (HCl) reacts with an antacid tablet containing 0.25 grams Al(OH) 3?
A) 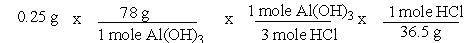
B) 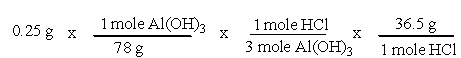
C) 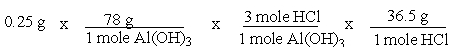
D) 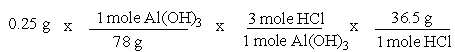
E) 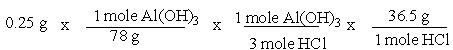
Correct Answer:

Verified
Correct Answer:
Verified
Q35: A chemical reaction involves many changes but
Q54: Pentane (C<sub>5</sub>H<sub>12</sub>) reacts with oxygen gas (O<sub>2</sub>)
Q55: The balanced equation for the combustion of
Q57: The following reaction is the formation of
Q59: How many moles is 9.1 <font face="symbol"></font>
Q60: Jane Doe has a cholesterol (C<sub>27</sub>H<sub>46</sub>O) count
Q61: During metabolism, table sugar (sucrose) is broken
Q62: Which of the following statements is the
Q63: Your friend combines vinegar (which contains acetic
Q72: Metabolism is<br>A) the breakdown of food.<br>B) the