Multiple Choice
The enthalpy for the reaction described by the energy diagram below is 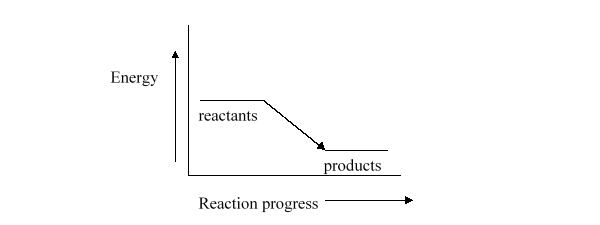
A) very large.
B) greater than zero, but not necessarily very large.
C) exactly zero.
D) less than zero.
E) It is not possible to predict anything about the enthalpy of this equation.
Correct Answer:

Verified
Correct Answer:
Verified
Q26: Bond breaking is always endothermic and yet
Q39: What are biological catalysts called?<br>A) proteins<br>B) nucleic
Q105: Which of the following transformations is a
Q106: Below are several statements. Which of these
Q107: Arsenic poisoning is a serious problem in
Q108: How many grams of iron are in
Q110: If Jane Doe has a blood carbon
Q111: The following chemical equation, the decomposition of
Q112: The reaction of water with ammonia is
Q114: An exothermic reaction is one that<br>A) has