Multiple Choice
The reaction between acetic acid (CH3COOH) and methanol (CH3OH) is given below, followed by a list of changes that could be made to the reaction. Which of these changes will result in the equilibrium shifting to the left? 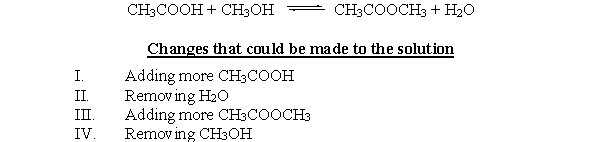
A) All of these changes will result in the equilibrium shifting to the left.
B) Only I will result in the equilibrium shifting to the left.
C) Only IV will result in the equilibrium shifting to the left.
D) I and III will result in the equilibrium shifting to the left.
E) III and IV will result in the equilibrium shifting to the left.
Correct Answer:

Verified
Correct Answer:
Verified
Q25: Under what circumstances is mass conserved?<br>A) Mass
Q33: How many atoms of oxygen are present
Q72: Where in the body does ethanol metabolism
Q73: An important part of metabolism is the
Q74: Which of the following statements describes how
Q75: Does one mole of the antibiotic penicillin
Q77: Which of the following do you expect
Q78: Arsenic poisoning is a serious problem in
Q79: How does increasing the temperature of a
Q80: In which figure are new O-H bonds