Multiple Choice
The reaction of water with ammonia is shown below. What will happen if ammonium is added to the solution when it is at equilibrium? 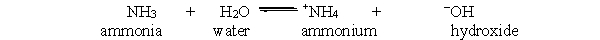
A) Nothing will happen.
B) The result is not predictable.
C) The ammonium will bubble.
D) The equilibrium will shift to the right.
E) The equilibrium will shift to the left.
Correct Answer:

Verified
Correct Answer:
Verified
Q60: In an exothermic reaction, the reactants are
Q83: One serving of animal crackers (30 g)
Q84: Which of the following characteristics of a
Q85: Which of the following molecules is known
Q86: Acetylene (C<sub>2</sub>H<sub>2</sub>) is a small organic molecule
Q87: The following chemical equation is not balanced.
Q89: Which of the following statements best describes
Q90: Which of the following reactions is balanced?<br>A)
Q92: Which bonds are broken over the course
Q93: The following reaction is a reversible reaction.