Multiple Choice
The average atomic mass of zirconium is 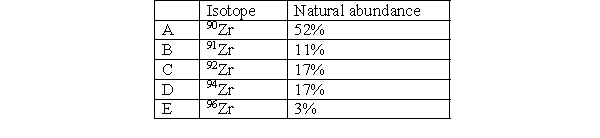
A) less than 90 because the atomic mass only depends on the number of protons in the atom.
B) 90 because 90Zn has the highest natural abundance.
C) greater than 90 but less than 96 because the atomic mass takes into account the abundance of all naturally occurring isotopes.
D) 96 because the atomic mass is the mass of the highest naturally occurring isotope.
E) greater than 96 because the atomic mass is the sum of masses of the naturally occurring isotopes.
Correct Answer:

Verified
Correct Answer:
Verified
Q4: How many electrons are in the valence
Q5: What is the most damaging electromagnetic radiation
Q6: _ is most commonly ingested along with
Q9: Iodine-131 has a half-life of eight days.
Q10: According to the graph, what is the
Q12: Which of the following is NOT a
Q13: Which of the imaging technique(s) listed below
Q50: What sort of protection should be used
Q69: Why is it necessary to shield yourself
Q88: The periods are the _ of the