Multiple Choice
During fatty acid oxidation, a fatty acid must react with coenzyme A to become a fatty acyl-CoA, as shown below. 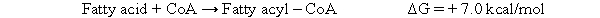 In the body, the reaction above is coupled to the hydrolysis of two phosphate groups from ATP as shown below:
In the body, the reaction above is coupled to the hydrolysis of two phosphate groups from ATP as shown below: 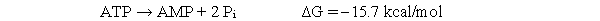 Which of the following equations is the correct calculation of the overall free energy of the coupled reaction?
Which of the following equations is the correct calculation of the overall free energy of the coupled reaction?
A) ΔGoverall = 7.0 kcal/mol + 15.7 kcal/mol
B) ΔGoverall = 7.0 kcal/mol 15.7 kcal/mol
C) ΔGoverall = 15.7 kcal/mol + 7.0 kcal/mol
D) ΔGoverall = 7 kcal/mol 15.7 kcal/mol
E) ΔGoverall = 7 kcal/mol 15.7 kcal/mol
Correct Answer:

Verified
Correct Answer:
Verified
Q35: Which of the following choices is NOT
Q81: Where in the cell does <font face="symbol"></font>-oxidation
Q82: During glucose metabolism, the following reaction occurs.
Q82: Which of the following choices illustrates the
Q83: The structure below is acetyl coenzyme A.
Q84: Which of the following statements best describes
Q85: Which of the following statements about spontaneous
Q87: Which of the choices above below describes
Q89: Why is the metabolism of fat sometimes
Q90: What is the identity of I in