Multiple Choice
Ascorbic acid, or vitamin C, is a water soluble vitamin. Which of the following interactions is primarily responsible for ascorbic acid's water solubility? 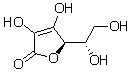
A) dispersion forces
B) dipole-dipole forces
C) hydrogen bonding forces
D) ionic bonding
E) covalent bonding
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q10: What are the products of the following
Q11: What is the product when this compound
Q13: What does [O] represent in the following
Q14: After an amide is hydrolyzed, it undergoes
Q16: What is the product of the following
Q17: The following reaction is the hydrogenation of
Q19: What type of reaction is this? <img
Q30: What is the purpose of NAD<sup>+</sup> in
Q32: Which food is NOT high in niacin?<br>A)
Q40: Which classification of alcohols can undergo oxidation