Multiple Choice
Is the aldehyde oxidized or reduced during the following reaction, and how can you tell? 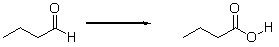
A) Neither. This is not an oxidation-reduction reaction.
B) The aldehyde is neither oxidized nor reduced because the number of hydrogens does not change.
C) The aldehyde is oxidized because it loses two hydrogens.
D) The aldehyde is reduced because it loses two hydrogens.
E) The aldehyde is oxidized because it gains an oxygen.
Correct Answer:

Verified
Correct Answer:
Verified
Q1: Which classification of alcohols cannot be oxidized,
Q15: In an oxidation-reduction reaction, the species that
Q76: During hydrolysis reactions, like all reactions, some
Q78: Which of the following statements describes coenzymes?<br>A)
Q78: When unsaturated fatty acids are hydrogenated, an
Q79: The following reaction shows the oxidation-reduction reaction
Q82: What is the product of the following
Q84: Which of the following species is NOT
Q85: Carbohydrates are molecules that are composed of
Q86: What is the product of the following