Multiple Choice
The two beakers below each have added to them the same amount of heat energy. Which statement best describes what would happen to the temperatures of the two beakers? 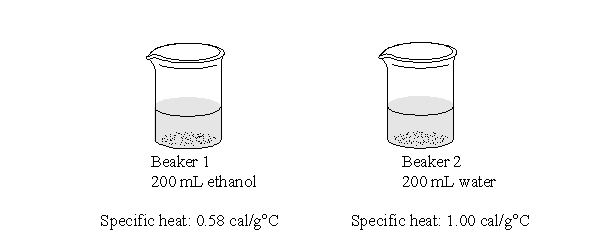
A) The temperature of the two beakers will remain the same.
B) The temperatures of the two beakers will increase by the same amount.
C) The temperature of beaker 1 will increase more than that of beaker 2.
D) The temperature of beaker 2 will increase more than that of beaker 1.
E) It is not possible to predict how the temperature of the beakers will change.
Correct Answer:

Verified
Correct Answer:
Verified
Q40: Consider a warm summer's day at the
Q41: Which of the following measurements is larger
Q42: Which of the following is most likely
Q44: The speed of molecules and atoms in
Q46: Tylenol is ordered for a child weighing
Q47: Chemistry attempts to explain the behavior of
Q48: Tylenol is ordered for a child weighing
Q49: How many mg of Tylenol should be
Q64: Which of the following describes the kinetic
Q88: Using significant figures, what is the sum