Multiple Choice
Galena is the ore from which elemental lead is extracted. In the first step of the extraction process, galena is heated in air to form lead(II) oxide. 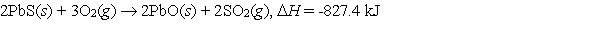 What mass of galena is converted to lead oxide if 975 kJ of heat are liberated?
What mass of galena is converted to lead oxide if 975 kJ of heat are liberated?
A) 203 g
B) 282 g
C) 406 g
D) 478 g
E) 564 g
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q15: The enthalpy (H) of liquid water is
Q44: Which one of the following equations
Q45: An important step in the synthesis
Q46: A backpacker collects snow at 0
Q47: Ethylene glycol, used as a coolant
Q50: 40.0 g of ice cubes at
Q51: and has work done on it
Q52: Calculate q when 28.6 g of
Q53: Which one of the following statements
Q54: What is the final temperature when