Multiple Choice
Calculate the enthalpy change for the reaction  from the following data:
from the following data: 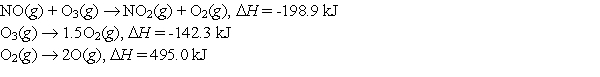
A) -551.6 kJ
B) -304.1 kJ
C) 190.9 kJ
D) 153.8 kJ
E) 438.4 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q7: For all processes, both q and w
Q27: Cold packs, whose temperatures are lowered when
Q32: Benzene is a starting material in
Q35: A system absorbs 21.6 kJ of heat
Q37: Calculate the <span class="ql-formula" data-value="\Delta"><span
Q38: A system expands from a volume of
Q39: An ideal gas (the system) is
Q40: A 275-g sample of nickel at
Q57: The standard heat (enthalpy) of formation of
Q82: A system delivers 1275 J of heat