Multiple Choice
Predict the products by completing a balanced equation for the following decomposition reaction. 
A) CaCl2(l) 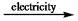 Ca(l) + 2Cl¯(l)
Ca(l) + 2Cl¯(l)
B) CaCl2(l) 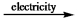 Ca2+(l) + Cl2(g)
Ca2+(l) + Cl2(g)
C) CaCl2(l) 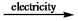 Ca2+(l) + 2Cl¯(l)
Ca2+(l) + 2Cl¯(l)
D) CaCl2(l)  Ca(l) + Cl2(g)
Ca(l) + Cl2(g)
E) CaCl2(l) 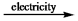 CaCl(l) + Cl¯(l)
CaCl(l) + Cl¯(l)
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q22: A standard solution of 0.243 M NaOH
Q34: Select the precipitate that forms when the
Q39: In a redox reaction, the oxidizing agent
Q43: In a redox reaction, the reducing agent
Q79: Calculate the oxidation number of the chlorine
Q79: A combination reaction may also be a
Q80: In both of the following reactions,
Q85: Select the net ionic equation for
Q90: Which of the following is a weak
Q104: The amount of calcium present in milk