Multiple Choice
Which one of the following equations correctly represents positron decay of 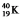 ?
?
A) 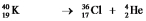
B) 
C) 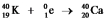
D) 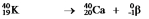
E) 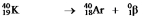
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: Write a complete, balanced equation to represent
Q36: Which one of the following elements is
Q50: In living organisms, C-14 atoms disintegrate at
Q54: A N-14 nucleus is hit by a
Q58: Radioactive decay follows zero-order kinetics.
Q68: Sodium-21 will emit positrons each having an
Q71: Most foodstuffs contain natural, radioactive isotopes.
Q73: A patient's thyroid gland is to
Q75: Which one of the following equations correctly
Q80: An isotope with Z > 83, which