Multiple Choice
The isotopes of promethium, 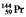 and
and 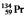 , are unstable, and lie on opposite sides of the "line of stability." Which of the following combinations is most likely to represent the type of decay for these isotopes?
, are unstable, and lie on opposite sides of the "line of stability." Which of the following combinations is most likely to represent the type of decay for these isotopes?
A) promethium-144, decay; promethium-134, positron decay
B) promethium-144, positron decay; promethium-134, decay
C) promethium-144, positron decay; promethium-134, electron capture
D) promethium-144, electron capture; promethium-134, positron decay
E) promethium-144, decay; promethium-134, decay
Correct Answer:

Verified
Correct Answer:
Verified
Q12: Identify the missing species in the following
Q13: The isotope <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7799/.jpg" alt="The isotope
Q14: The isotope <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7799/.jpg" alt=" The
Q19: Identify the missing species in the following
Q20: refer to which of the following particles?<br>A)
Q21: Which of the following isotopes is most
Q22: Explain how the number of protons and
Q55: Bombardment of uranium-238 nuclei by carbon-12 nuclei
Q62: Detection of radiation by a Geiger-Müller counter
Q62: Which of the following types of radioactive