Multiple Choice
Consider the following octahedral complex structures, each involving ethylene diamine and two different, unidentate ligands X and Y. 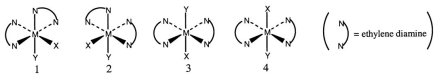 Which one, if any, of the following is a pair of optical isomers?
Which one, if any, of the following is a pair of optical isomers?
A) 1 and 2
B) 1 and 3
C) 1 and 4
D) 3 and 4
E) None of these choices is correct.
Correct Answer:

Verified
Correct Answer:
Verified
Q8: Of the 3d transition series of elements,
Q19: When the ethylenediaminetetraacetate ion (EDTA<sup>4-</sup>) forms a
Q20: Which of the following species could exist
Q21: In the formation of a transition metal
Q25: What is the highest possible oxidation state
Q33: The conversion of the chromate ion (CrO<sub>4</sub><sup>2-</sup>)
Q34: A characteristic of ligands is that<br>A)they are
Q36: The Cu<sup>2+</sup> ion has 1 unpaired electron.
Q41: What is the highest possible oxidation state
Q86: Mercury(II) compounds are assimilated into the food