Multiple Choice
Consider the following octahedral complex structures, each involving ethylene diamine and two different, unidentate ligands X and Y. 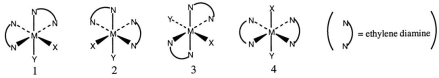 Which one of the following statements about these structures is incorrect?
Which one of the following statements about these structures is incorrect?
A) Structures 1 and 2 are optical isomers.
B) Structures 1 and 3 are optical isomers.
C) Structures 1 and 3 are different complexes.
D) Structures 1 and 4 are geometrical isomers.
E) Structures 3 and 4 are the same complex.
Correct Answer:

Verified
Correct Answer:
Verified
Q7: The inner transition series of elements arise
Q14: Which one of the following has the
Q21: Which of the following ions could exist
Q43: The M<sup>2+ </sup>ions of the first transition
Q48: Aluminum reacts with oxygen in the air
Q49: In the compound [Ni(en)<sub>2</sub>(H<sub>2</sub>O)<sub>2</sub>]SO<sub>4</sub> (where en =
Q51: Which of the following will be paramagnetic?<br>A)V
Q62: A feature of transition metal chemistry is
Q71: Which of the following is not a
Q74: The most common oxidation state for ions