Multiple Choice
Consider the following structures (1 and 2 are octahedral; 3 and 4 are square planar) . 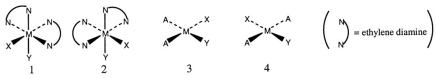 Which one of the following statements about the above structures is correct?
Which one of the following statements about the above structures is correct?
A) 1 and 2 are superimposable.
B) 1 and 2 are geometric isomers.
C) 3 and 4 are structural isomers.
D) 3 and 4 are optical isomers.
E) 3 and 4 are geometric isomers.
Correct Answer:

Verified
Correct Answer:
Verified
Q17: A certain transition metal complex has the
Q20: A certain transition element has the stable
Q37: Which of the following transition elements can
Q60: Tetrahedral complexes can exhibit both optical and
Q76: Give the systematic name for [CoCl<sub>3</sub>(H<sub>2</sub>O)]¯.<br>A) cobalt(II)
Q77: Chromium and manganese are among the transition
Q78: Which of the following is considered a
Q81: A) State the requirement for two molecules
Q84: A) How many unpaired 3d electrons will
Q85: Which of the following ligands could participate