Essay
Potassium is produced industrially by the reaction Na(l) + K+(l) 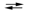 Na+(l) + K(g). The reaction is carried out at 850 C, but the equilibrium Kc constant strongly favors the reactants. Explain how the conditions are manipulated to ensure a good yield of potassium despite the small value of Kc.
Na+(l) + K(g). The reaction is carried out at 850 C, but the equilibrium Kc constant strongly favors the reactants. Explain how the conditions are manipulated to ensure a good yield of potassium despite the small value of Kc.
Correct Answer:

Verified
The gaseous potassium product ...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q5: Transition elements from the left side of
Q11: Lightning, fires and other atmospheric processes result
Q16: The bond energy in the hydrogen molecule
Q16: Compared to the asbestos diaphragm-cell used in
Q17: Which of the following reactions is
Q19: The alkali metals are isolated from non-aqueous
Q32: Nitrogen fixation occurs through atmospheric, industrial, and
Q35: The most common source for commercial production
Q40: Plants extract phosphate from the soil<br>A)by converting
Q41: The kinetic isotope effect is the basis