Multiple Choice
Which one of the following is the best representation of the titration curve which will be obtained in the titration of a weak diprotic acid H2A (0.10 mol L¯1) with a strong base of the same concentration?
A) 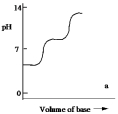
B) 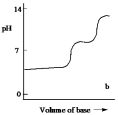
C) 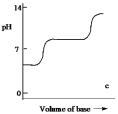
D) 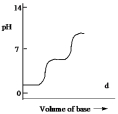
E) 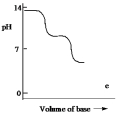
Correct Answer:

Verified
Correct Answer:
Verified
Q17: The solubility of magnesium phosphate is 2.27
Q19: A phosphate buffer (H<sub>2</sub>PO<sub>4</sub>¯/HPO<sub>4</sub><sup>2</sup>¯) has a pH
Q21: What will be the effect of adding
Q23: A lab technician adds 0.20 mol of
Q24: What is the maximum amount of sodium
Q25: A 20.0-mL sample of 0.30 M HClO
Q26: Calculate the solubility of silver oxalate, Ag<sub>2</sub>C<sub>2</sub>O<sub>4</sub>,
Q27: Which of the following aqueous mixtures would
Q64: When 20.0 mL of 0.15 M hydrochloric
Q73: Equal volumes of the following pairs of