Multiple Choice
A diprotic acid H2A has Ka1 = 1 *10¯4 and Ka2 = 1 * 10¯8. The corresponding base A2¯ is titrated with aqueous HCl, both solutions being 0.1 mol L¯1. Which one of the following diagrams best represents the titration curve which will be seen?
A) 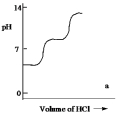
B) 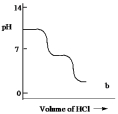
C) 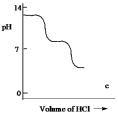
D) 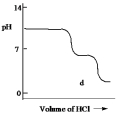
E) 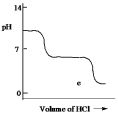
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q22: A buffer is prepared by adding 150
Q81: A lab technician adds 0.015 mol of
Q82: Calculate the solubility of lead(II) iodide, PbI<sub>2</sub>,
Q84: A 20.0-mL sample of 0.25 M HNO<sub>3</sub>
Q85: Buffer solutions with the component concentrations shown
Q87: A buffer is prepared by adding 1.00
Q88: The lab technician Anna Lytic adds 2.20
Q89: What is the maximum mass of KCl
Q91: An acetic acid buffer containing 0.50 M
Q106: When a weak acid is titrated with