Multiple Choice
Write the ion product expression for calcium phosphate, Ca3(PO4) 2.
A) 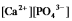
B) 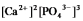
C) 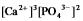
D) 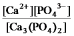
E) None of these choices is correct.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q33: Increasing the concentrations of the components of
Q37: If the pH of a buffer solution
Q68: At the equivalence point in an acid-base
Q76: Calculate the solubility of silver chromate, Ag<sub>2</sub>CrO<sub>4</sub>,
Q79: The salts X(NO<sub>3</sub>)<sub>2</sub> and Y(NO<sub>3</sub>)<sub>2</sub> (where X<sup>+</sup>
Q81: A lab technician adds 0.015 mol of
Q82: Calculate the solubility of lead(II) iodide, PbI<sub>2</sub>,
Q84: A 20.0-mL sample of 0.25 M HNO<sub>3</sub>
Q85: Buffer solutions with the component concentrations shown
Q106: When a weak acid is titrated with