Multiple Choice
Consider the dissolution of MnS in water (Ksp = 3.0 * 10¯14) . 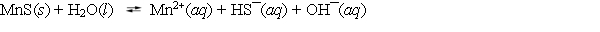 How is the solubility of manganese(II) sulfide affected by the addition of aqueous potassium hydroxide to the system?
How is the solubility of manganese(II) sulfide affected by the addition of aqueous potassium hydroxide to the system?
A) The solubility will be unchanged.
B) The solubility will decrease.
C) The solubility will increase.
D) The amount of KOH added must be known before its effect can be predicted.
E) The pKa of H2S is needed before a reliable prediction can be made.
Correct Answer:

Verified
Correct Answer:
Verified
Q14: The solubility of salt MX (solubility product
Q22: A buffer is prepared by adding 150
Q53: A solution is prepared by adding 100
Q88: The lab technician Anna Lytic adds 2.20
Q89: What is the maximum mass of KCl
Q91: An acetic acid buffer containing 0.50 M
Q92: The indicator propyl red has K<sub>a</sub> =
Q94: The salts X(NO<sub>3</sub>)<sub>2</sub> and Y(NO<sub>3</sub>)<sub>2</sub> (where X<sup>+</sup>
Q96: A 20.0-mL sample of 0.50 M H<sub>2</sub>C<sub>6</sub>H<sub>6</sub>O<sub>6</sub>
Q106: A CH<sub>3</sub>COOH/CH<sub>3</sub>COO- buffer can be produced by