Multiple Choice
The acid dissociation constant Ka equals 1.26 *10¯2 for HSO4¯ and is 5.6 *10¯10 for NH4+. Which statement about the following equilibrium is correct? 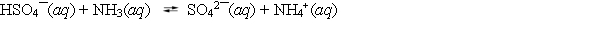
A) The reactants will be favored because ammonia is a stronger base than the sulfate anion.
B) The products will be favored because the hydrogen sulfate ion is a stronger acid than the ammonium ion.
C) Neither reactants nor products will be favored because all of the species are weak acids or bases.
D) The initial concentrations of the hydrogen sulfate ion and ammonia must be known before any prediction can be made.
E) This reaction is impossible to predict, since the strong acid and the weak base appear on the same side of the equation.
Correct Answer:

Verified
Correct Answer:
Verified
Q25: Which of the following liquids contains the
Q33: The strongest base which can exist in
Q35: It is not possible to have a
Q65: A solution is prepared by adding 0.10
Q66: The pH of a 0.200 M solution
Q67: What is the pH of a 0.0035
Q68: Formic acid, HCOOH, is a weak acid
Q72: According to Brønsted and Lowry, which one
Q78: A solution is prepared by adding 0.10
Q79: Hydrated metal ions in aqueous solution can