Multiple Choice
Nitrogen dioxide can dissociate to nitric oxide and oxygen. 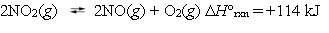 Under which reaction conditions would you expect to produce the largest amount of oxygen?
Under which reaction conditions would you expect to produce the largest amount of oxygen?
A) high temperature, high pressure
B) low temperature, high pressure
C) high temperature, low pressure
D) low temperature, low pressure
E) None of these choices is correct, unless a catalyst is present
Correct Answer:

Verified
Correct Answer:
Verified
Q29: SO<sub>2</sub> reacts with O<sub>2</sub> to produce SO<sub>3</sub>.
Q92: The equilibrium constant, K<sub>p</sub>, has a value
Q93: Hydrogen sulfide will react with water as
Q94: (p. Various sections) Which of the following
Q95: Hydrogen sulfide can be formed in the
Q96: The equilibrium constant for the reaction of
Q97: The equilibrium constant for reaction (1) below
Q99: Write the mass-action expression, Q<sub>c</sub>, for the
Q100: Consider the equilibrium reaction shown below. B<sub>2</sub>(g)
Q102: At 25 <span class="ql-formula" data-value="\degree"><span