Essay
Briefly outline the key arguments in the collision theory of reaction rates for the elementary reaction 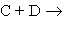 products. Show that this theory predicts a second-order rate law, and how it predicts the form of the rate constant k.
products. Show that this theory predicts a second-order rate law, and how it predicts the form of the rate constant k.
Correct Answer:

Verified
For reaction to occur, the molecules mus...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q18: Is a bimolecular reaction necessarily second-order? Is
Q23: Consider the following mechanism for the oxidation
Q25: The rate law for the rearrangement of
Q26: The rate law for the reaction
Q28: Dinitrogen tetraoxide, N<sub>2</sub>O<sub>4</sub>, decomposes to nitrogen
Q31: The compound RX<sub>3</sub> decomposes according to
Q32: A reaction has the following rate law:
Q48: A transition state is a species (or
Q54: If the activation energy of a reaction
Q54: According to the collision theory of reaction