Multiple Choice
Select the correct Lewis structure for nitrogen trifluoride, NF3.
A) 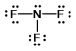
B) 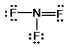
C) 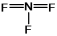
D) 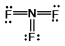
E) 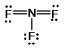
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q9: Which one of the following molecules contains
Q15: In which one of the following species
Q62: According to VSEPR theory, a molecule with
Q76: Draw Lewis structures, showing all valence electrons,
Q77: Hydrazine, N<sub>2</sub>H<sub>4</sub>, is a good reducing agent
Q79: Phosphoryl iodide is used in the preparation
Q80: Draw Lewis structures, showing all valence electrons,
Q82: Bond angles of 180 <span
Q83: Predict the actual bond angles in
Q85: The formal charges on Cl and O