Multiple Choice
Select the Lewis structure for XeO2F2 which correctly minimizes formal charges.
A) 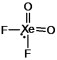
B) 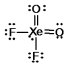
C) 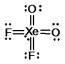
D) 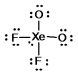
E) 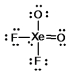
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q17: The molecule AX<sub>2</sub>, where A and X
Q22: Using SO<sub>2</sub> as an example, describe the
Q54: In neutral molecules, how many bonds are
Q54: Use VSEPR theory to predict the electron
Q55: What is the molecular shape of NH<sub>2</sub>Cl
Q88: Which of the following has no net
Q90: In carbon disulfide, how many lone pairs
Q94: Predict the ideal bond angles in
Q96: Select the best Lewis structure for P<sub>2</sub>I<sub>4</sub>.<br>A)
Q100: Predict the actual bond angle in