Multiple Choice
In the following Lewis structure for phosphate, phosphorus has a formal charge of ____ and an oxidation number of ____. 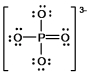
A) 0, -3
B) 0, 5
C) 5, -3
D) 5, 5
E) 3, 5
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q7: Boron never achieves an octet in any
Q20: In order for a noncyclic triatomic molecule
Q43: How many faces and how many vertexes
Q63: What is the molecular shape of ClCN
Q64: Considering all the bonds in a
Q69: What is the molecular shape of ClF<sub>4</sub><sup>¯</sup>
Q70: List all possible molecular geometries (shapes) for
Q72: Thionyl chloride is used as an oxidizing
Q73: What is the molecular shape of SiF<sub>6</sub><sup>2¯</sup>
Q89: The best Lewis structure for sulfuric acid