Multiple Choice
In which of the following does the nitrogen atom have a formal charge of -1?
A) 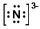
B) 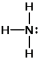
C) 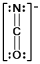
D) 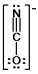
E) 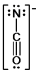
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q5: What is the molecular shape of the
Q7: Predict the ideal bond angles around
Q8: What is the molecular shape of NOCl
Q9: What is the molecular shape of XeO<sub>2</sub>F<sub>2</sub>
Q11: In which one of the following is
Q15: Which one of the following Lewis structures
Q42: In the COCl<sub>2</sub> molecule, carbon is the
Q55: According to VSEPR theory, a molecule with
Q68: In the nitrate ion (NO<sub>3</sub><sup>−</sup>), nitrogen and
Q96: According to VSEPR theory, a molecule with