Essay
A Lewis structure of aspirin without the nonbonding electrons is shown in the figure below.Identify the hybridization of the C1 and C2 atomic orbitals.Arrange the bonds (B1-B5) in order of increasing length. 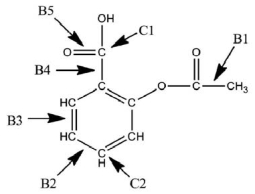
Correct Answer:

Verified
C1 and C2 are sp2 h...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q55: Ethanol has the formula CH<sub>3</sub>CH<sub>2</sub>OH.The oxygen atom
Q56: Which compound below has the largest dipole
Q57: Cyclohexane (C<sub>6</sub>H<sub>12</sub>) contains carbon atoms that
Q58: Which molecule below has a carbon atom
Q59: What type of hybridization is needed to
Q61: What is the valence electron molecular
Q62: Ethanol has the formula CH<sub>3</sub>CH<sub>2</sub>OH.The central carbon
Q63: Which compound below has a square pyramidal
Q64: Which type of molecular orbital is
Q65: Draw the Lewis structure of BrF<sub>5</sub>.Give the