Multiple Choice
The relative energies (strengths) of the intermolecular forces between five different substances are shown in the figure below.Which substance has the lowest boiling point? 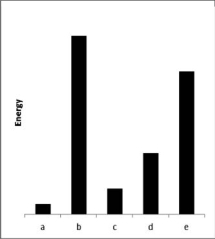
A) a
B) b
C) c
D) d
E) e
Correct Answer:

Verified
Correct Answer:
Verified
Q30: Which gaseous compound below is likely
Q31: When an ion is dissolved in water,
Q32: Based on their boiling points,which compound below
Q35: Which statement below is FALSE?<br>A)The more electrons
Q36: Which molecule below has the highest polarizability?<br>A)N<sub>2</sub><br>B)O<sub>2</sub><br>C)F<sub>2</sub><br>D)Br<sub>2</sub><br>E)I<sub>2</sub>
Q37: Which compound below will exhibit hydrogen bonding
Q38: Which pair of compounds below is most
Q39: For a molecule to exhibit dipole-dipole interactions,
Q57: At the critical point, _<br>A) all the
Q86: Molecular nitrogen (N<sub>2</sub>) interacts with water and