Multiple Choice
The relative energies (strengths) of the intermolecular forces present in each of five different pure substances are shown in the figure below.Which substance has the highest melting point? 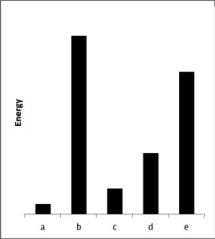
A) a
B) b
C) c
D) d
E) e
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q25: Describe the similarity and the difference between
Q48: Which alcohol should be most soluble in
Q68: The relative energies (strengths) of the intermolecular
Q69: Sketch the hydrated ions that form when
Q70: You are interested in determining the solubility
Q73: Which compound below has the highest boiling
Q74: Hydrophilic substances _<br>A)are immiscible in water.<br>B)are insoluble
Q75: Which compound below is likely to have
Q76: Point c in the phase diagram below
Q77: The solubility of a compound may depend