Multiple Choice
Polymers with ionic functional groups have been developed for removal of ions from water.One example is Amberlite.One form of Amberlite has the structure shown below, where the -SO3H groups act like a weak acid.What type of ions will this polymer attract? 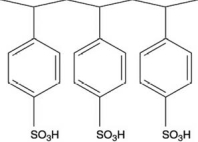
A) cations
B) anions
C) all ions
D) impossible to tell
E) none, since the polymer is neutral
Correct Answer:

Verified
Correct Answer:
Verified
Q34: Which species is oxidized in the
Q35: What is the formula of the precipitate
Q36: Silver salts are used in black-and-white photography.Ag<sup>+</sup>
Q37: Limestone (CaCO<sub>3</sub>, 100.09 g/mol) is present in
Q40: How many grams of iron(III) hydroxide (106.87
Q41: Write the balanced molecular equation and the
Q43: Write the net ionic equation for
Q44: Identify which of the following ionic compounds
Q126: A homogeneous mixture of two or more
Q128: Oxidation refers to _<br>A)an increase in oxidation