Essay
Give the formula for the precipitate that will form when the following aqueous solutions are mixed.If no precipitate forms, write "none." 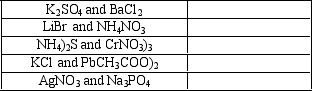
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q43: Write the net ionic equation for
Q44: Identify which of the following ionic compounds
Q45: A 25.00 mL sample of H<sub>2</sub>SO<sub>4</sub> requires
Q46: What mass of silver chloride will be
Q48: Potassium is a very reactive metal, but
Q49: Approximately 192 grams of ammonium nitrate
Q50: Calculate parts per million Br<sup>-</sup> in a
Q52: In which of the following does manganese
Q84: Magnesium metal burns brightly in the air
Q126: A homogeneous mixture of two or more