Multiple Choice
Which statement regarding the boiling of a mixture of liquids A and B is NOT correct? 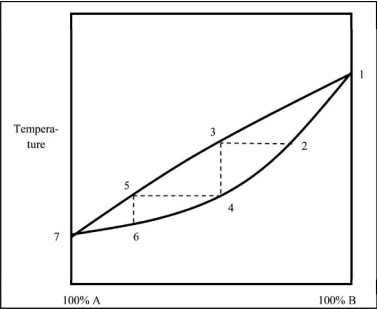
A) The distillate collected boils at lower temperatures as the percentage of A increases.
B) The distillate always contains a higher percentage of B because pure B is less volatile than pure A.
C) The upper curve reflects the composition of the vapor at a given temperature.
D) As the distillation progresses from point 2 to point 6, the liquid becomes richer in A.
E) The boiling point of pure A is lower than that of pure B.
Correct Answer:

Verified
Correct Answer:
Verified
Q93: Identify the following statement as true or
Q112: A 0.512 g sample of an
Q113: How does the presence of solutes raise
Q114: Lanterns and stoves that use n-pentane
Q116: A solution is prepared by mixing
Q118: In the process of dialysis, a special
Q119: What is the vapor pressure of
Q120: The normal boiling point of ammonia
Q121: Which statement below regarding the liquid-gas phase
Q122: At 25 <span class="ql-formula" data-value="\degree"><span