Multiple Choice
Which of the following statements regarding the phase diagram of water and an aqueous solution is NOT correct? Temperature is on the x-axis; pressure is on the y-axis. 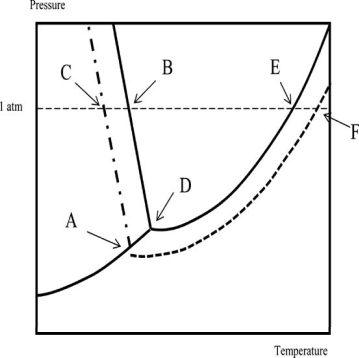
A) The boiling point of the solution is higher than that of the solvent by an amount indicated by the difference in temperature between points E and F.
B) Point D corresponds to the triple point of the solvent.
C) The solution boils at the temperature corresponding to point F.
D) The normal freezing point of the solution corresponds to point A.
E) The freezing point of the solvent is higher than that of the solution.
Correct Answer:

Verified
Correct Answer:
Verified
Q4: A solution of 5.00 g of lithium
Q9: Two solutions, A and B, are separated
Q10: The Henry's law constant for oxygen
Q12: A solution is prepared by mixing
Q13: A solution contains pentane (C<sub>5</sub>H<sub>12</sub>, 72.15
Q15: What is the osmotic pressure of
Q16: Myristicin is a hallucinogenic compound found
Q17: The concentration unit of molality is symbolized
Q18: The normal boiling point of bromine
Q68: Seawater can be characterized by the following