Short Answer
Henry's law constant for oxygen dissolving in blood is 3.74 *10-2 mol/L . atm at body temperature, 37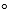 C.Calculate the molar concentration of oxygen in blood for a free diver where the air pressure is 3.0 atm.The mole fraction of oxygen in air is 0.209.
C.Calculate the molar concentration of oxygen in blood for a free diver where the air pressure is 3.0 atm.The mole fraction of oxygen in air is 0.209.
Correct Answer:

Verified
2.3 * 10View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q1: What physical property is used to separate
Q52: A 15.0 mg sample of a protein
Q100: Gasoline is primarily a mixture of
Q101: The vapor pressure of an aqueous
Q102: Define the term "colligative" in relation to
Q103: Which solution, if either, would create the
Q103: Indicate which aqueous solution has the lowest
Q105: A solution is prepared by adding
Q107: The normal freezing point of benzene
Q108: You wish to de-ice your driveway, so