Multiple Choice
The following titration curve is most likely to be associated with 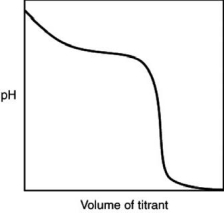
A) the titration of a strong acid with a strong base titrant.
B) the titration of a weak acid with a strong base titrant.
C) the titration of a strong base with a strong acid titrant.
D) the titration of a weak base with a strong acid titrant.
Correct Answer:

Verified
Correct Answer:
Verified
Q10: If 500 mL of a solution containing
Q11: What is the molar concentration of Ag<sup>+</sup>(aq)
Q12: Which of the following compounds does NOT
Q13: At the equivalence point of a strong
Q14: What is the pH of a solution
Q16: Suppose a 1.0 L solution containing 0.20
Q17: Calculate the equilibrium concentration of ZN<sup><sub>2</sub> +
Q18: Vitamin C is a monoprotic weak acid,
Q19: Chromium(III) hydroxide shows greater solubility in strongly
Q20: The solubility product for CaCO<sub>3</sub> is written