Essay
Based on the information in the table of standard reduction potentials below, explain what, if anything, will happen if iodine is placed in an aqueous solution containing bromide ions. 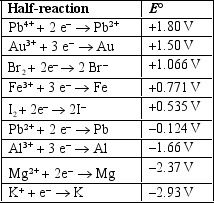
Correct Answer:

Verified
No reaction will occur. I2 has ...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q125: The standard hydrogen electrode is<br>A)used to calibrate
Q126: Reduction refers to<br>A)loss of mass.<br>B)an increase in
Q127: Using the following data, determine E<sup>o</sup><sub>cell
Q128: A 1.5 V alkaline battery is constructed
Q129: Identify the strongest reducing agent in the
Q130: Glancing at a periodic table, where do
Q131: The spontaneous redox reaction in a
Q133: The standard cell potential for the
Q134: What is the cell potential for
Q135: The following reaction is called the