Short Answer
Cesium 137 (Cs137)is a short-lived radioactive isotope.It decays at a rate proportional to the amount of itself present and has a half-life of 30 years (i.e., the amount of Cs137 remaining t years after A0 mg of the radioactive isotope is released is given by 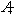 ).As a result of its operations, a nuclear power plant releases Cs137 at a rate of 0.1 mg per year.The plant began its operations in 1990, which we will designate as t = 0.Assume there is no other source of this particular isotope.In the long run, approximately how many mg of Cs137 will there be? Round to 2 decimal places.
).As a result of its operations, a nuclear power plant releases Cs137 at a rate of 0.1 mg per year.The plant began its operations in 1990, which we will designate as t = 0.Assume there is no other source of this particular isotope.In the long run, approximately how many mg of Cs137 will there be? Round to 2 decimal places.
Correct Answer:

Verified
Correct Answer:
Verified
Q18: Let x be the number of
Q19: In an electrical circuit, a damping
Q20: Is <span class="ql-formula" data-value="y=x^{2}+x"><span class="katex"><span
Q21: The rate of change of a
Q22: Solve <span class="ql-formula" data-value="\frac {
Q24: Let x be the number of
Q25: Solve the initial value problem and
Q26: The slope field for the differential
Q27: Suppose that the function P(t)satisfies the
Q28: An artist works on a museum