Multiple Choice
Consider the following list of electron configurations: 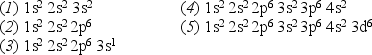
-Which one of the above configurations represents an excited state of a neutral atom?
A) 1
B) 2
C) 3
D) 4
E) 5
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q18: Which one of the following electronic configurations
Q19: Why was it necessary for Bohr to
Q20: An atom will emit photons when one
Q21: The electron in a hydrogen atom is
Q22: Consider the following list of electron configurations:
Q24: Determine the maximum number of electron states
Q25: What is the total number of subshells
Q26: Which one of the following will result
Q27: Consider the following list of electron configurations:
Q28: Each atom in the periodic table has