Multiple Choice
A 200.0-kg object is attached via an ideal pulley system to paddle wheels that are submerged in 0.480 kg of glycerin at 20.0 °C in an insulated container as shown.Then, the object falls through a distance of 5.00 m causing the paddle wheel to turn.Assuming all of the mechanical energy lost by the falling object goes into the water, determine the final temperature of the glycerin.The specific heat capacity of glycerin is 2410 J/ 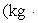 Co) .
Co) . 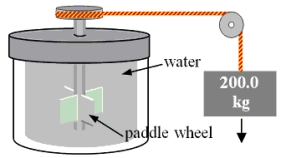
A) 4.90 °C
B) 28.5 °C
C) 24.9 °C
D) 40.4 °C
E) 8.47 °C
Correct Answer:

Verified
Correct Answer:
Verified
Q4: During an evening news broadcast in Helsinki,
Q5: An ordinary mercury thermometer at room temperature
Q16: Which one of the following would probably
Q20: The specific heat capacity of iron is
Q38: A 2.0-g sample of steam at 100
Q40: Which one of the following temperatures is
Q41: Heat is added to a 1.0-kg solid
Q46: A 2.00-kg metal object requires 1.00 ×
Q49: What is the minimum amount of energy
Q63: Complete the following statement: When a substance