Multiple Choice
Heat is added to a 1.0-kg solid sample of a material at -200 °C. The figure shows the temperature of the material as a function of the heat added. 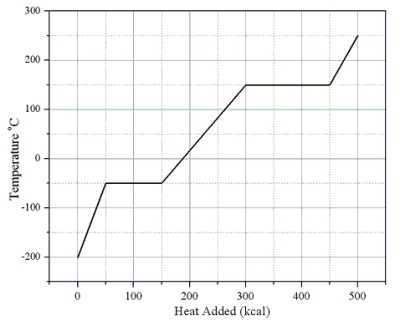
-What is the specific heat capacity of this substance in its liquid state?
A) 0.33 cal/(g · C°)
B) 0.75 cal/(g · C°)
C) 1.00 cal/(g · C°)
D) 1.33 cal/(g · C°)
E) 3.00 cal/(g · C°)
Correct Answer:

Verified
Correct Answer:
Verified
Q36: The units of heat are equivalent to
Q37: Using the data in the table, determine
Q38: A 2.0-g sample of steam at 100
Q39: Determine the latent heat of vaporization of
Q40: Which one of the following temperatures is
Q42: Absolute zero on the Celsius temperature scale
Q43: A circular hole in an copper plate
Q44: The digital sign outside a local bank
Q45: Heat is added to a 1.0-kg solid
Q46: A 2.00-kg metal object requires 1.00 ×