Multiple Choice
Two deuterium nuclei, 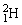 , fuse to produce a tritium nucleus,
, fuse to produce a tritium nucleus, 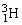 , and a hydrogen nucleus. A neutral deuterium atom has a mass of 2.014102 u; a neutral tritium atom has a mass of 3.016050 u; a neutral hydrogen atom has a mass of 1.007825 u; a neutron has a mass of 1.008665 u; and a proton has a mass of 1.007277 u. How much energy is released in the process? 1 u = 931.494 MeV/c2.
, and a hydrogen nucleus. A neutral deuterium atom has a mass of 2.014102 u; a neutral tritium atom has a mass of 3.016050 u; a neutral hydrogen atom has a mass of 1.007825 u; a neutron has a mass of 1.008665 u; and a proton has a mass of 1.007277 u. How much energy is released in the process? 1 u = 931.494 MeV/c2.
A) 3.03 MeV
B) 3.53 MeV
C) 4.03 MeV
D) 4.53 MeV
E) 6.58 MeV
Correct Answer:

Verified
Correct Answer:
Verified
Q31: Which of the following is not used
Q32: The reaction <sup>2</sup>H + <sup>2</sup>H → <sup>3</sup>H
Q33: What kind of reactor produces more fissionable
Q34: How does the total mass of the
Q35: Find the Q value of the following
Q37: Which of the following best describes the
Q38: All of the following are units used
Q39: Consider the fission reaction: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3817/.jpg" alt="Consider
Q40: How much energy is released in the
Q41: PET is an abbreviation for<br>A)photon-electron tunneling.<br>B)positron emission