Multiple Choice
The image shows bonding for diamond on the left) and graphite on the right) . Both minerals are made of carbon, so why do they have very different properties why is diamond so hard while graphite is so soft) ? 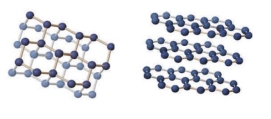
A) Covalent bonds in both minerals are very strong, but intermolecular bonds between sheets in graphite are very weak.
B) Graphite's mixture of covalent and intermolecular bonds is stronger than the solely covalent bonds within diamond.
C) Diamond has a different type of covalent bonds between the atoms of carbon than does graphite.
D) Minerals with only one type of bond will always be harder than minerals that have two different types of bonding.
Correct Answer:

Verified
Correct Answer:
Verified
Q43: If a mineral cleaves into thin sheets,
Q44: From the list provided below, choose those
Q45: What characteristic of water helps it cause
Q46: Using the Periodic Table, what can you
Q47: What type of silicate minerals is shown
Q49: Which of the following is NOT a
Q50: What type of cleavage is illustrated in
Q51: What type of rock is shown in
Q52: Magnetite and hematite occur together in layered
Q53: What three kinds of particles are the