Multiple Choice
Which of the following compounds would be expected to be more soluble in hexane (C6H14) ?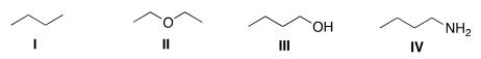
A) I
B) II
C) III
D) IV
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q2: What intermolecular force is generally considered the
Q6: Which of the following molecules can bond
Q7: Which of the following statements best describes
Q8: Which of the following structures contains an
Q9: Which of the following molecules contain the
Q12: Which of the following statements about vitamin
Q13: Which of the following compounds has the
Q14: Rank the following compounds in order of
Q16: Which of the following correctly matches the
Q38: Which of the following lists contains common