Multiple Choice
Would this crossed Aldol reaction work well? Why or why not?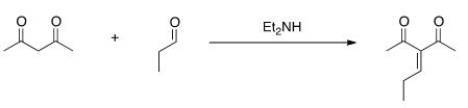
A) Yes, the diketone is significantly more acidic, so this enolate can be formed selectively.
B) Yes, the aldehyde is significantly more acidic, so this enolate can be formed selectively.
C) No, the aldehyde is significantly more acidic, so this enolate cannot be formed selectively.
D) No, the diketone is significantly more acidic, so this enolate cannot be formed selectively.
Correct Answer:

Verified
Correct Answer:
Verified
Q1: <sub> </sub>Under basic conditions, the Aldol reaction
Q3: What is the Aldol addition product formed
Q4: What is the Aldol addition product formed
Q5: <sup> </sup>The b-hydroxy carbonyl product of an
Q6: The following reaction is an example of
Q7: <sup> </sup>What cyclic product is formed in
Q9: Which of the following bicyclic ring systems
Q10: <sub> </sub>There are several variations of the
Q11: <sub> </sub>Of the carbonyl compounds; (1) benzaldehyde,
Q42: What is the general name for the